
HCC Biomarkers
Information for Health Care Providers
The HCC Biomarker tests AFP-L3 and DCP are in vitro diagnostic blood tests for the risk assessment of developing Hepatocellular Carcinoma (HCC). Both tests are used in conjunction with imaging studies, clinical assessment and other laboratory findings and are CE-certified. They are run on the µTASWakoTM i30 device.
In Europe, about 70% of all cases of Hepatocellular Carcinoma (HCC) are diagnosed at an advanced stage and curative treatments are only available for a minority of patients. Therefore, optimal surveillance of at-risk patients is crucial for the early detection of HCC.
The current guideline from the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC) recommends that patients with a high risk of liver cancer should be screened at 6-month intervals using abdominal ultrasound1.However, ultrasound is highly operator dependent and its performance is limited when used on patients who are obese or have a severe background liver cirrhosis.
Several studies have shown that AFP-L3 and DCP are complementary markers for liver cancer. HCCs are biologically not homogeneous. Some express normal levels of AFP, but high levels of AFP-L3 or DCP and vice versa. The combined use of these three markers provides higher clinical sensitivity and is effective for the early recognition of HCC2/3.
HCC surveillance improves patient outcome
The GALAD score improves diagnostic performance
Investigators around Prof. Johnson from the UK established an algorithm, in which gender, age, AFP-L3, AFP and DCP are calculated. This so-called GALAD score can detect early stages of HCC at a sensitivity of at least 75% and a specificity of 89%7.
Recently, the GALAD model was validated in an international setting by analyzing nearly 7,000 datasets from the UK, Germany, Japan and Hong Kong. AUROC analysis in all cohorts showed better values for GALAD compared to individual or combined markers. The model performance did not vary between the etiologies HBV, HCV and “others”. It even performed well in small and early HCC with AUC values of at least 0.85 and could successfully discriminate between HCC and other hepatobiliary cancers8.
Liver cancer surveillance
Screening tests such as ultrasonography and blood tests at regular intervals are crucial to identify liver cancer at an early stage in individuals who are at risk. This allows the application of effective curative treatments at an early stage which are associated with better long-term outcomes.
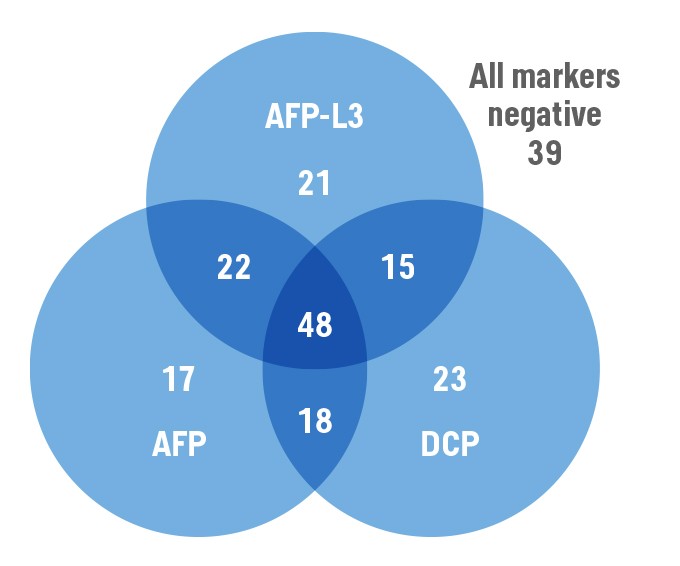
Distribution of HCC patients with various patterns of positivity for the HCC biomarkers3
The determination of the markers allows the serological detection of tumors in early stages even if the AFP shows negative results. In a broad investigation of 270 HCC patients who all had a serum AFP of less than 20 ng/ml, the combined use of the biomarkers detected 49% of all patients with tumors with the size of less than 2 centimetres.4
Thus, adding AFP-L3 and DCP to HCC surveillance can increase the chances of detecting early HCC and therefore, should be incorporated into regular surveillance practices. The HCC management guideline of the Japan Society of Hepatology includes all three serum biomarkers for surveillance5. A 2010 survey report on surveillance efficacy in Japan concluded that out of all patients with HCC, 33.5% had tumors of 2 centimetres or less and more than 62% had undergone surgery or local ablation therapy which represent treatment options that are only available for early stage HCC6.
The BALAD-2 score enables a prognosis assessment
The British research group established another model called BALAD-2. It includes markers of liver disease severity (bilirubin and albumin) and markers of tumor biology (AFP, AFP-L3 and DCP). BALAD-2 analyses assign patients into four distinct prognostic groups from relatively best to worst survival9.
Key Features
- AFP-L3 and DCP tests are CE-certified
- Combined use of AFP, AFP-L3 and DCP increases the sensitivity of HCC detection
- Markers increase the chance of detecting HCC early of patients at risk
- GALAD score specifically detects early HCC at high sensitivity nearly irrespective of etiology
References
1 EASL and EORTC, EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma, Journal of Hepatology, April 2012, 56 (4): 908-943
2 Ertle J. et al., A Combination of α-fetoprotein and Des-γ-carboxy Prothrombin is Superior in Detection of Hepatocellular Carcinoma, Digestion 2013, 87 (2): 121-31
3 Ohmura et al., Annual Meeting of Japanese Society of Laboratory Medicine, 2009
4 Toyoda H. et al., Clinical Utility of Highly Sensitive Lens Culinaris Agglutinin-reactive α-fetoprotein in Hepatocellular Carcinoma Patients with α-fetoprotein <20 ng/ml, Cancer Science, May 2011, 102 (5): 1025-1031
5 Makuuchi M. et al., Development of Evidence-based Clinical Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma in Japan, Hepatology Research, January 2008, 38 (1): 37-51
6 Ikai I. et al., Report of the 18th Follow-up Survey of Primary Liver Cancer in Japan, Hepatology Research, October 2010, 40 (11): 1043-1059
7 Johnson P. et al., The Detection of Hepatocellular Carcinoma using a Prospectively Developed and Validated Model Based on Serological Biomarkers, Cancer Epidemiology, Biomarkers & Prevention, January 2014, 23 (1): 144-153
8 Berhane S. et al., Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients, Clinical Gastroenterology and Hepatology, June 2016, 14 (6); 875-886
9 Fox R et al., Biomarker-based Prognosis in Hepatocellular Carcinoma: Validation and Extension of the BALAD model, British Journal of Cancer, April 2014, 110 (8): 2090-2098
Newsletter
Subscribe to the free newsletter and ensure that you will no longer miss any offers or news of FUJIFILM Wako Chemicals Europe GmbH.



