
β-Glucan Test – Information for Health Care Providers
The β-Glucan Test is an in vitro diagnostic test for the quantitative determination of (1→3)-β-D-glucan in serum or plasma. It is a helpful marker of many invasive fungal infections.
The assay is performed on the LIMUSAVE MT-7500 or Toxinometer MT-6500 device.
Invasive fungal diseases are a significant worldwide health problem, and their prevalence is increasing. These opportunistic infections affect immunocompromised patients, those undergoing intensive-care treatment and people with chronic disorders, in particular lung diseases. Invasive fungal diseases are important causes of morbidity and mortality and difficult to diagnose. Members of the two genera Aspergillus1, Candida2 and the species Pneumocystis jirovecii3 cause the majority of these infections by far. The early recognition and diagnosis of mycoses is of outstanding importance for improving patient outcomes. However, traditional diagnostic tools such as pathologic histological and fungal cultures lack the sensitivity and capacity needed for early diagnoses.
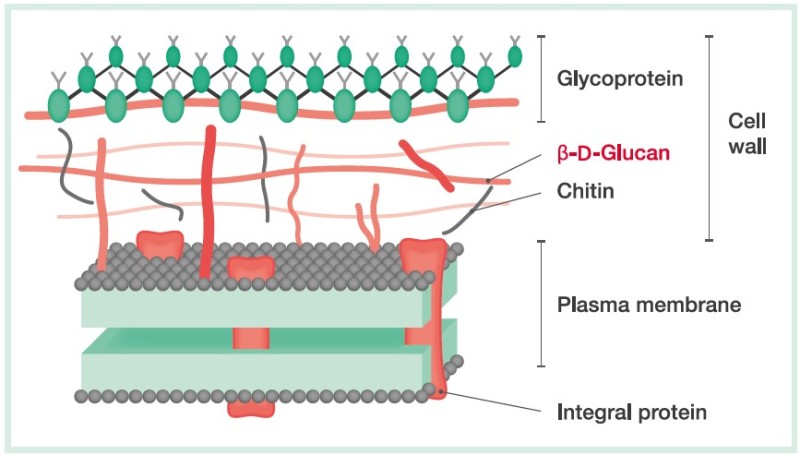
(1→3)-β-D-Glucan measurement in invasive fungal infections
In most pathogenic fungi, (1→3)-β-D-glucan is an integral component of the cell wall (Fig. 1). Small quantities are released into the blood during infection. The Limulus reagent (LAL: Limulus amebocyte lysate), made from the extract of blood cells of horseshoe crabs, has drawn attention as an in vitro diagnostic reagent for mycosis. It reacts with (1→3)-β-D-glucan as well as with endotoxin. The β-Glucan Test exclusively measures the (1→3)-β-D-glucan concentration in serum or plasma through a kinetic turbidimetric assay.
Clinical diagnostic performance
The literature describes high diagnostic efficacies using the turbidimetric β-Glucan Test (table 1).
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| Invasive aspergillosis | 80.0% | 97.3% (182/187) | 86.5% (32/37) | 95.8% (182/190) |
| Candidiasis | 98.7% (77/78) | 97.3% (182/187) | 93.9% (77/82) | 99.5% (182/183) |
| Pneumocystis pneumonia | 94.1% (16/17) | 97.3% (182/187) | 76.2% (16/21) | 99.5% (182/183) |
Supply throughout the EMEA region thanks to our distributors
Key Features
- Single test reagent
- Calibration by QR code scan
- Quality control available
- Simple procedure thanks to ready-to-use reagents and intuitive software
- Quantitative β-glucan measurement by the kinetic turbidimetric method
Downloads
References
1Ullmann A.J. et al., Diagnosis and Management of Aspergillus Diseases: Executive Summary of the 2017 ESCMID-ECMM-ERS Guideline. Clinical Microbiology and Infection 24 (2018)
2Calandra T. et al., Diagnosis and Management of Invasive Candidiasis in the ICU: An Updated Approach to an Old Enemy. Critical Care (2016) 20:125
3Sokulska M. et al., Pneumocystis jirovecii--From a Commensal to Pathogen: Clinical and Diagnostic Review. Parasitol Res. 2015 Oct;114(10):3577-85
4De Carolis et al., Comparative performance evaluation of Wako β-glucan test and Fungitell assay for the diagnosis of invasive fungal diseases. PLoS One 2020 Jul 29;15(7).
Please visit the Mediacenter for the latest publications and further information.
β-Glucan Test – FUJIFILM Wako products
Hauptmerkmale
- Der ß-Glucan Test ist CE-zertifiziert
- Hohe Sensitivität und Spezifität für die meisten invasiven Pilzpathogene
- Früherkennung der Infektion erleichtert therapeutische Entscheidungen
- Monotest-Verfahren für schnelle Messungen in Krankenhäusern
Newsletter
Subscribe to the free newsletter and ensure that you will no longer miss any offers or news of FUJIFILM Wako Chemicals Europe GmbH.


